Background: There are few studies currently available on the prevention of GVHD by mesenchymal stem cells (MSCs) that use a systematic approach to prevent both aGVHD and cGVHD simultaneously. The clinical investigation of MSCs preventing either aGVHD or cGVHD independently is the main focus of the earlier studies.
Aim:Using the strategy of co-transplantation of MSCs and HSCs and phased application of MSCs after transplantation (MSCs 100-day scheme), we seek to develop a secure and dependable system that can avoid both aGVHD and cGVHD based on our preliminary research.
Method:Patients undergoing haploidentical adult hematopoietic stem cell transplantation will receive unaltered, fourth-generation UC-MSCs(Umbilical Cord). In addition to the usual GVHD preventive measures, UC-MSCs are given four hours before the transplantation of peripheral blood stem cells on day 0. UC-MSCs are administered once weekly for 1-4 weeks, once every 2-8 weeks, and once every 4-12 weeks after transplantation. The dose needed for each transfusion is 1 10 6/kg. Our research will look at how well UC-MSCs prevent GVHD following haplo-HSCT, as well as recurrence rates after transplantation, transplant-related issues, and the state of immunological reconstruction. the frequency, severity, 1- and 2-year survival rates, cumulative death rates, and the occurrence of aGVHD and cGVHD.Real-time tracking will be performed to monitor the occurrence of aGVHD and cGVHD, as well as their severity, frequency, 1-year and 2-year survival rates, and cumulative mortality rates. The analysis of the differences between the control group and the experiment will take into account the pre-transplant ferritin levels, CMV serological status of the donor-recipient, blood type matching situation, HLA locus matching situation, disease remission status, residual disease conditions, recipient age, donor-recipient gender matching situation, stem cell quantities (nucleated cells and CD34+ cell counts), and stem cell numbers (nucleated cells and CD34+ cell counts).
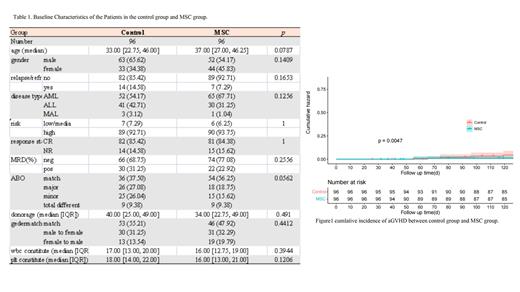
Results: 96 patients from the MSCs group and 96 patients from the control group are now accessible for statistical purposes. Demographic factors for age and gender distribution do not differ considerably. Acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndrome-EB2, and acute mixed cell leukemia,which were all classified as having the same level of risk. The cumulative incidence of aGVHD grades II to IV is 19.8% in the MSCs group, while grades III to IV is 0%. Comparable indications for the control group are 43.8% and 9.3%, respectively. The overall incidence of aGVHD differs between the two groups in a statistically significant way (P=0.0047). The rates of moderate and severe cGVHD in the control group were 10.4% and 9.4%, respectively, whereas they were 13.5% and 4.2% in the MSCs group. Over the course of 1 year, the cumulative incidence of cGVHD for the MSCs group was 36.5%, compared to 39.6% for the control group. Regarding the overall two-year OS, there is no discernible difference between the MSCs group and the control group (P=0.43).While the MSCs group outperformed the control group in terms of GRFS (GVHD free and relapse free survival).
Conclusion: The strategy of MSCs 100-day scheme infusion can successfully lower the incidence of acute and chronic GVHD and is beneficial for enhancing patient GRFS.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal